
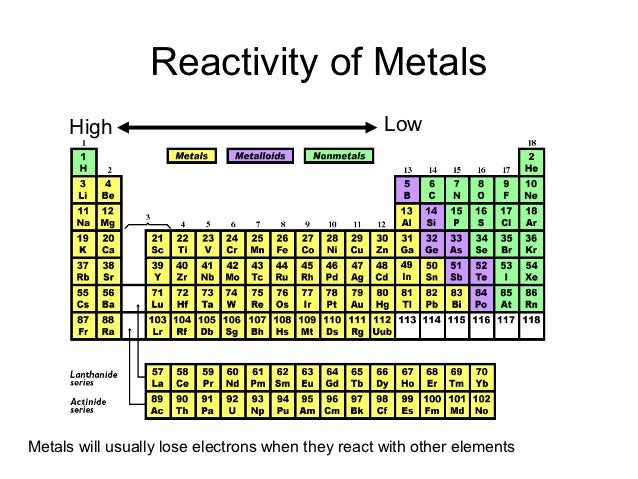
Everything in the middle is somewhere in between. Non-reactive elements like those on the right of the periodic table have nice happy families of electrons that don't need to make any new friends. Highly reactive elements like the ones on the left side of the periodic table have a lonely electron flying around by itself so it wants to make friends and react with the electrons from other atoms. It might help to think of electrons as social little creatures. The electrons on the other hand fly around the nucleus in a big cloud. The neutrons and protons are bundled up in the nucleus or center of the atom. The reason that different elements have different levels of reactivity has to do with the structure of the atoms themselves.Īn atom is made of protons, neutrons and electrons. Understanding all the reasons behind the various intermediate reactivities is where the career in chemistry comes in. All the rest of the elements in between are less than perfectly stable, so they react with each other to various degrees. For instance, fluorine (9) and chlorine (17) are short one electron from being the most stable and they are very reactive! Sodium (11) and Potassium (19) have extra electrons and are very reactive. The most reactive elements tend to be short one or two electron or have one or two extra. All other atoms have less stable electron numbers, so they react with each other and share electrons to be more stable. They have a stable number of electrons and do not need to react with other atoms. If you look at a periodic table, you see that these numbers correspond to the right hand side - the noble gases - these are very NOT reactive. When an atom has particular numbers of electrons around it, it is more stable - 2, 10, 18, etc.
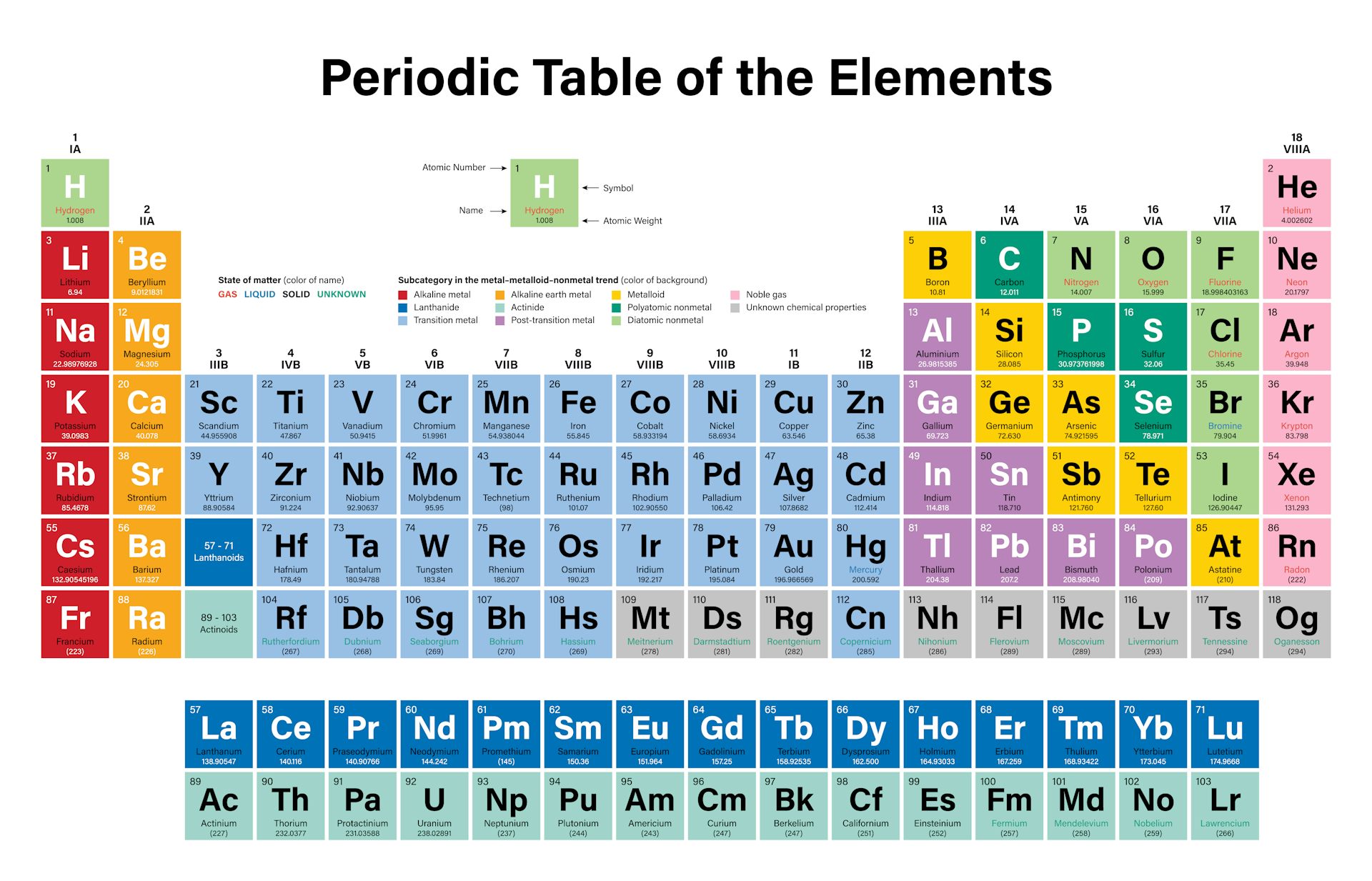
Electrons do not orbit exactly the way the moon orbits the earth, but if you think of it that way to start with, it works.Īll chemical reactions occur between the electrons of different atoms. The neutrons and protons are in the center of the atom and the electrons "orbit" around the outside. Have you studied the structure of an atom yet?Īn atom is composed of neutrons, protons, and electrons.
Periodic table reactivity full#
This is a great question and a full answer would take a career in chemistry, but I'll try to give you a shorter answer.

Why are some of the periodic table elements reactive?


 0 kommentar(er)
0 kommentar(er)
